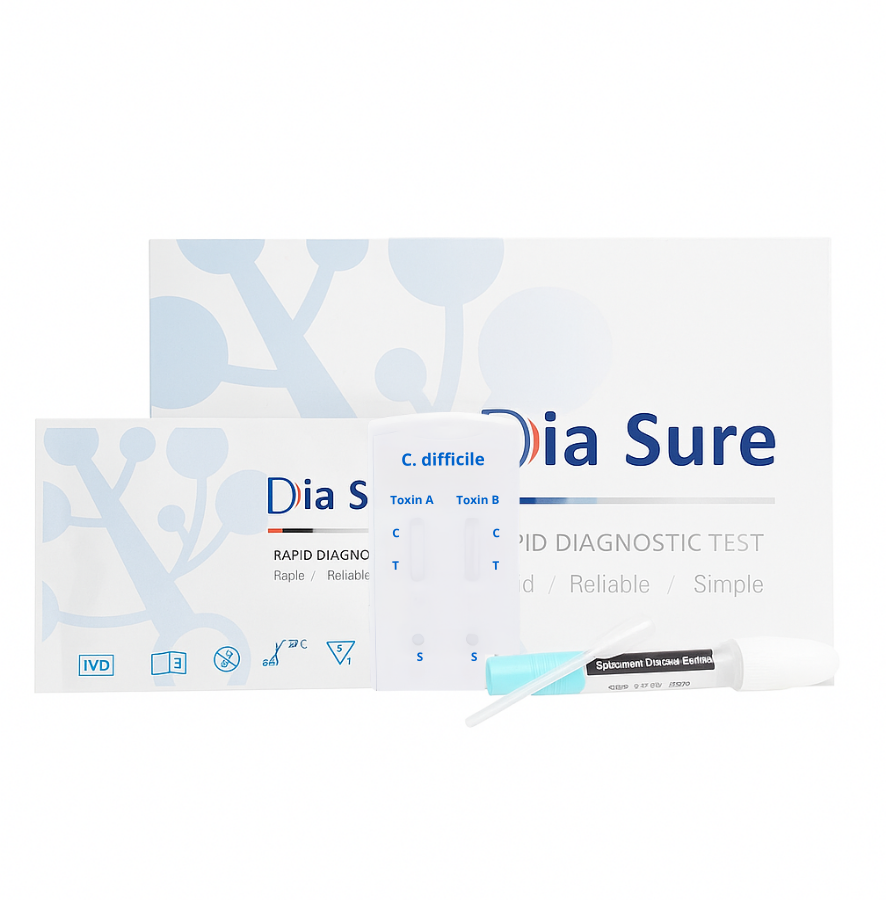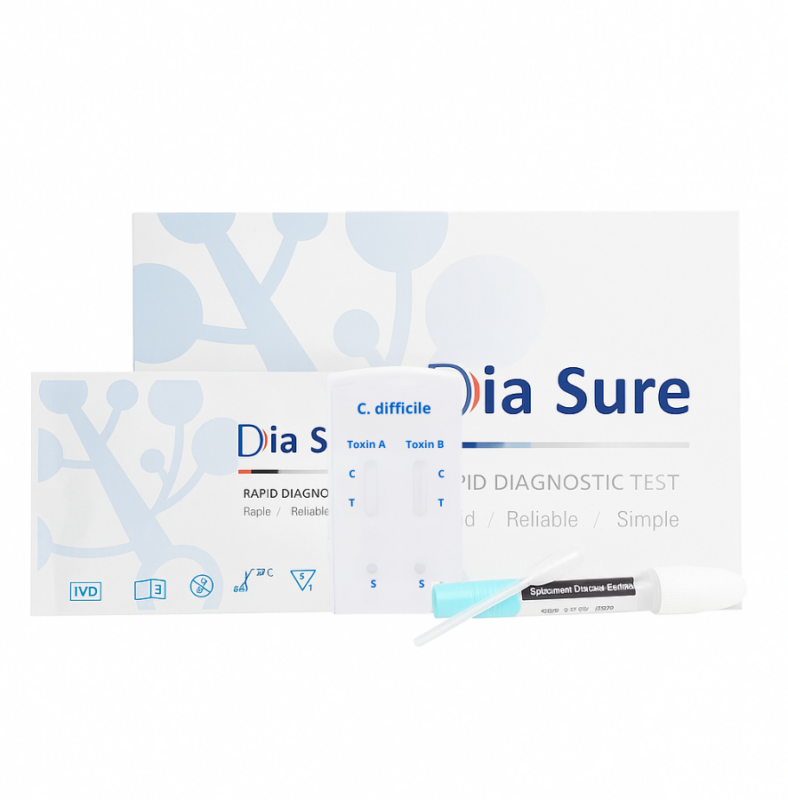
Rapid Test for Detection of C. difficile Toxins A/B in Stool (CDF-F42)
A highly sensitive in vitro immunochromatographic rapid test designed for the qualitative detection of Clostridium difficile Toxin A and Toxin B in human fecal samples.
Ease of use, rapid results (within 10 minutes), and no requirement for specialized laboratory equipment make this test kit an effective tool for the initial diagnosis of C. difficile infections caused by toxigenic strains, in hospitals, clinics, laboratories, and field settings.
The test provides high accuracy and enables separate detection of both Toxin A and Toxin B. Results should be interpreted in the context of clinical presentation and confirmed by additional laboratory methods when needed.
TEST KIT CONTENTS
Test Kit Contents
Individually packed test devices (cassettes)
Package insert (instructions)
Disposable pipettes
Dilution tube with buffer for sample preparation

TEST PROCEDURE

Before use, bring the tests, samples, buffer, and/or controls to room temperature (15–30°C).
Sample collection and preparation
Use the sample collection and dilution tube included in the kit. Follow the instructions on the label. Other clean and dry containers may also be used. Best results are obtained if the analysis is performed within 6 hours after collection.
For solid samples: unscrew the applicator of the buffer tube. Be careful not to spill the contents. Insert the applicator into at least 3 different parts of the stool to collect approximately 120 mg.
For liquid samples: hold the pipette vertically, aspirate the sample, and transfer 3 drops into the buffer tube.
Return the applicator to the tube and tightly close the cap. Do not break the tip. Shake the tube thoroughly to fully mix the sample with the buffer.
Samples may be stored at –20°C for up to 6 months if not tested within 1 hour after preparation.
Test procedure
1. Remove the test from the pouch and place it on a clean, flat surface. Label the test (patient or control).
2. Break the tip of the tube (e.g., using a tissue). Hold vertically and dispense 2 drops into the sample well (S). Avoid air bubbles. Do not drop into the result window.
3. Once the test starts, color will begin to move along the membrane. Wait 10 minutes. Results read after 20 minutes are considered invalid.
INTERPRETATION OF RESULTS

POSITIVE
Two colored lines appear on the membrane: one in the control region (C), and one in the test region (T).

NEGATIVE
Only one colored line appears in the control region (C). No line appears in the test region (T).



 Blood Gas and Electrolyte Analyzers
Blood Gas and Electrolyte Analyzers